Congenital Heart Disease in children | classification and Overview
This guide covers the classification of congenital heart disease in children with an overview of each category and will set the stage to understand individual congenital heart disease.
The initial evaluation for suspected CHD involves a systematic approach with three major components:
Understanding the classification of congenital heart disease is an essential first step in deciding the diagnostic approach towards them since the classification is mostly based on pathophysiology and alteration of hemodynamics. The final diagnosis can then be confirmed by echocardiography, CT or MRI, or cardiac catheterization.
Classification of congenital heart diseases
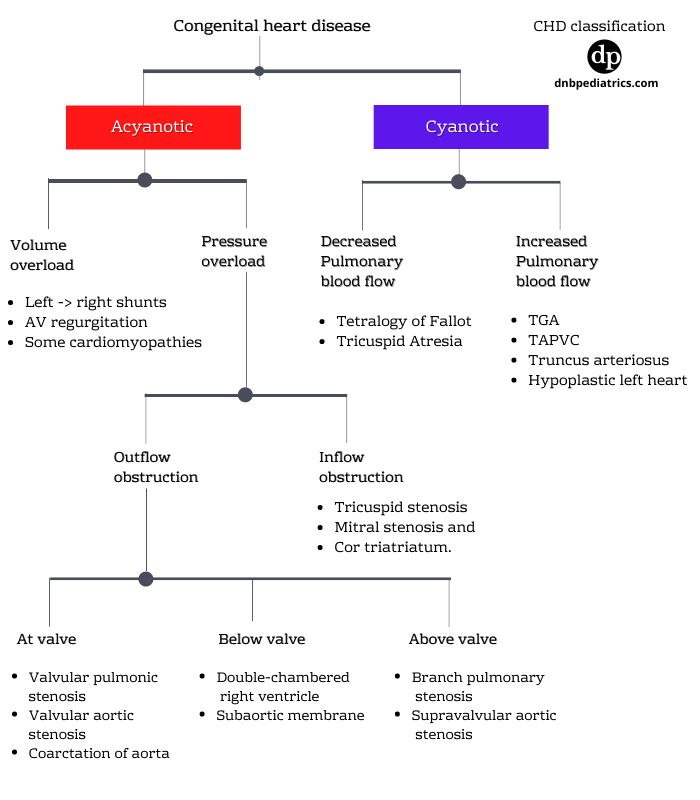
Let us start with a CHD presenting without cyanosis
1. Acyanotic heart diseases
Acyanotic heart diseases can be classified according to the predominant physiologic load that they place on the heart. In other words, whether they cause...
- Volume overload or
- Pressure overload
Acyanotic heart disease with Volume overload
a. left-to-right shunt lesions like ASD, VSD or PDA
b. Atrioventricular valve regurgitation
c. some of the cardiomyopathies
Acyanotic heart disease with Pressure overload
Thus they can be further classified as
- Secondary to ventricular outflow obstruction such as pulmonary or aortic valve stenosis or
- Narrowing of one of the great vessels such as coarctation of the aorta.
Conditions with Volume overload
A. Left to Right shunts
These are the most common lesions in this group.
- Atrial septal defect
- ventricular septal defect (VSD)
- AV septal defects (AV canal)
- Patent ductus arteriosus (PDA)
The pathophysiologic common denominator in this group is communication between the systemic and pulmonary sides of the circulation, which results in shunting of fully oxygenated blood back into the lungs.
The shunt can be quantitated by calculating the ratio of pulmonary (Qp) to systemic blood flow (Qs) termed usually as Qp: Qs.
A 2:1 shunt implies twice the normal pulmonary blood flow.
The direction and magnitude of the shunt across such communication depend on
- The size of the defect
- The relative pulmonary and systemic pressure gradient and vascular resistance
• Since the vascular resistance offered by the pulmonary bed is lower than that offered by the systemic bed, the direction of flow is from systemic (left) to pulmonary (right).
• This increased blood flow across the pulmonary bed results in pulmonary congestion and recurrent respiratory tract infections.(RRTI). With long-standing and major shunt, the alteration occurs in the pulmonary bed causing increasingly higher resistance and the shunts eventually may reverse as the pulmonary pressure may supersede the systemic pressures.
Mechanism of symptoms in left to right shunts
• The pulmonary bed is not prepared to receive more than physiological blood flow which increased hydrostatic pressure. Fluid leaks into the interstitial space and alveoli causing pulmonary edema.
• The increased volume of blood in the lungs decreases pulmonary compliance and increases the work of breathing.
• This leads to symptoms such as tachypnea, chest retractions, nasal flaring, and wheezing.
• The term heart failure in Left to right shunt is usually a misnomer. Total left ventricular output is actually several times greater than physiological, although much of this output is ineffective because it returns (shunts) directly to the lungs without being utilized systemically.
• To maintain this high level of left ventricular output, heart rate and stroke volume are increased adding to tachycardia, and increased WOB. This is mediated by an increase in sympathetic nervous system activity. (Raised catecholamines)
• The increase in circulating catecholamines along with the increased work of breathing causes an elevation in total body oxygen consumption, often beyond the oxygen-carrying ability of the circulatory system.
Such a high oxygen consumption leads to the additional symptoms of sweating, irritability, and failure to thrive.
In long run, this results in remodeling of the heart, with predominantly dilatation and a lesser degree of hypertrophy.
What is Eisenmenger physiology?
If left untreated, pulmonary vascular resistance eventually begins to rise, and, by several years of age, the shunt volume will decrease and eventually reverse to right to left which is called Eisenmenger physiology.
B. Regurgitant lesions
Regurgitation through the AV valves is most commonly encountered in patients with partial or complete AV septal defects (anteroseptal defects, AV canal).
In these lesions, the combination of a left-to-right shunt with AV valve regurgitation increases the volume load on the heart and leads to more severe symptoms.
Isolated regurgitation through the tricuspid valve is seen in the Ebstein anomaly.
Regurgitation involving one of the semilunar valves is usually also associated with some degree of stenosis.
However, aortic regurgitation may be encountered in patients with a VSD directly under the aortic valve (supracristal VSD) and in patients with membranous subaortic stenosis.
C. Cardiomyopathies
Cardiomyopathies may affect systolic contractility or diastolic relaxation, or both. Decreased cardiac function results in increased atrial and ventricular filling pressure, and Increased filling pressure ultimately causes pulmonary edema.
The major causes of cardiomyopathy in infants and children include
- Viral myocarditis,
- Metabolic disorders, and
- Genetic defects.
- Idiopathic
Conditions with pressure overload
The pathophysiologic common denominator of these lesions is an obstruction to normal blood flow.
Obstructions to ventricular outflow - More common
Ventricular outflow obstruction can occur
At the valve
- Valvular pulmonic stenosis
- Valvular aortic stenosis
- Coarctation of the aorta
Below the valve
- Double-chambered right ventricle
- Subaortic membrane
Above the valve
- branch pulmonary stenosis
- Supravalvular aortic stenosis
Obstruction to ventricular inflow - Less common
- Tricuspid stenosis
- Mitral stenosis and
- Cor triatriatum.
Unless the obstruction is severe, cardiac output will be maintained and the clinical symptoms of heart failure will be either subtle or absent.
This compensation predominantly involves an increase in cardiac wall thickness (hypertrophy), but in later stages it also involves dilatation.
2. Cyanotic congenital heart diseases
This group of congenital heart disease predominantly presents with cyanosis. They are further divided according to pathophysiology. See Image above.
A. Cyanotic lesions with decreased pulmonary blood flow
This type must include
- An obstruction to pulmonary blood flow ( right ventricular or pulmonary valve level)
- A pathway by which systemic venous blood can be shunted from right to left and enter the systemic circulation via a PFO, ASD, or VSD.
Common lesions in this group
- Tricuspid atresia
- Tetralogy of Fallot
- Various forms of single ventricle with pulmonary stenosis
This group can be further classified based on restrictive and non-restrictive intra-cardiac shunt
| Cyanotic heart disease with decreased PBF - Non-restrictive | Cyanotic heart disease with decreased PBF - Restrictive |
| 1. TOF 2. Tetralogy of Fallot (with PS+Non restrictive VSD 3. Pulmonary atresia with Non-restrictive VSD | 1. Pulmonary atresia + ASD without VSD 2. PS+ASD+PFO 3. Tricuspid atresia+PS+restrictive ASD 4. Ebstein's anomaly 5. Trilogy of Fallot |
In these groups, the degree of cyanosis depends on the degree of obstruction to pulmonary blood flow.
Patients with obstructive cyanotic heart disease may have hypercyanotic (“tet”) spells during the conditions of stress.
If the obstruction is severe, pulmonary blood flow may be dependent on the patency of the ductus arteriosus. When the ductus closes in the 1st few days of life, the neonate experiences profound hypoxemia and shock which is why the duct has to be kept patent by using pharmacological or surgical interventions.
B. Cyanotic lesions with increased pulmonary blood flow
This group is not associated with obstruction to pulmonary blood. Cyanosis is caused by either abnormal mixing of venous and arterial blood. Deoxygenated systemic venous blood and oxygenated pulmonary venous blood mix completely in the heart and, as a result, oxygen saturation is equal in the pulmonary artery and aorta.
Transposition of the great vessels (TGA) is the most common of the former group of lesions.
Total mixing lesions include cardiac defects with a common atrium or ventricle, total anomalous pulmonary venous return, and truncus arteriosus.
If pulmonary blood flow is not obstructed, these infants have a combination of cyanosis and heart failure due to volume overload. In contrast, if pulmonary stenosis is present, these infants have cyanosis alone, similar to patients with the tetralogy of Fallot.
How to approach congenital heart disease?
Approach to a newborn with suspected heart disease (pdf)
Want to read more on cardiology?
Author

Ranjith C S. | DNB (Pediatrics), DM (Medical Oncology)
Ranjith has completed Pediatric Residency from Kanchi Kamakoti Childs Trust Hospital and further trained in Medical Oncology from JIPMER
💡 Join the Discussion!
🩺 Help us refine this article — share corrections or additional information below. Let's elevate the accuracy of knowledge together! 💉💬